Scientists are searching for the secret in Doug Whitney’s biology that has protected him from dementia, hoping it could lead to ways to treat or prevent Alzheimer’s for many other people.
Before dawn on a March morning, Doug Whitney walked into a medical centre 3200 kilometres from home, about to transform from a mild-mannered, bespectacled retiree into a superhuman research subject.
First, a doctor inserted a needle into his back to extract cerebral spinal fluid — “liquid gold”, a research nurse called it, for the valuable biological information it contains. Then, the nurse took a sample of his skin cells. After that came an injection of a radioactive tracer, followed by a brain scan requiring him to lie still for 30 minutes with a thermoplastic mask over his face. Then, another tracer injection and another brain scan.
During his three-day visit to the centre, at Washington University School of Medicine in St Louis, he also had cognitive assessments, neurological evaluations and blood draws that extracted multiple tubes for analysis.
For 14 years, Whitney has been the one-person focus of exceptionally detailed scientific investigation, for which he travels periodically to St Louis from his home in Port Orchard, Washington. It is not because he is ill. It is because he was supposed to be ill.
Whitney, 76, is a scientific unicorn with the potential to provide answers about one of the world’s most devastating diseases. He has a rare genetic mutation that essentially guaranteed he would develop Alzheimer’s disease in his late 40s or early 50s and would likely die within a decade.
His mother and nine of her 13 siblings developed Alzheimer’s and died in the prime of their lives. So did his oldest brother, and other relatives going back generations. It is the largest family in the United States known to have an Alzheimer’s-causing mutation.
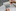
“Nobody in history had ever dodged that bullet,” Whitney said.
But somehow, he has done just that. Something has shielded him from his genetic destiny, allowing him to escape Alzheimer’s for at least 25 years longer than anyone expected.
Scientists are searching for the recipe for his biological secret sauce. Its discovery could lead to medications or gene therapies to prevent, treat or possibly even cure Alzheimer’s.
“This is an amazing case,” said Dr Kenneth Kosik, a neuroscientist at the University of California, Santa Barbara, who is not part of Whitney’s research team. “There are huge implications in the answers and in posing the questions.”
Alzheimer’s afflicts about 7 million Americans and about 32 million people worldwide. In most cases, the direct cause is unknown and symptoms begin after age 65.
About 1% of cases, however, are known to be caused by one of three genetic mutations. Inheriting one of those almost always causes early-onset Alzheimer’s, which often progresses quickly toward death.
Because genetic early-onset Alzheimer’s closely resembles typical late-onset Alzheimer’s, studying these families can yield important insights.
“Almost everything we know about Alzheimer’s today comes from these rare mutations,” Kosik said.
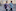
Whitney’s family has the rarest mutation, Presenilin 2. Mutation carriers in Whitney’s family usually began exhibiting memory and thinking problems between ages 44 and 53.
When Whitney turned 50, his wife, Ione, said that she and their two children began watching for signs.
When he reached 55, the age his mother and brother died, his family’s antennae became even more attuned.
“‘How’s Dad doing?’” their son and daughter asked whenever they called home.
“‘I don’t see anything,’” Ione Whitney replied.
“When he turned 60,” she recalled, “it was like, ‘We are good.’”
Then a cousin, Gary Reiswig, contacted them saying that he was writing a book about the family and that researchers were seeking more members of families with early-onset Alzheimer’s mutations to study.
Doug Whitney agreed to participate and to undergo genetic testing, assuming that he did not have the mutation. But on his 62nd birthday, he learned that he did.
“I was speechless,” he said. “I mean, I was at least 10 or 12 years past when I should have got sick.”
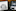
Dr Randall Bateman, a neurologist who directs the Dominantly Inherited Alzheimer Network, known as DIAN, at Washington University, was stunned, too.
“We tested him three different times,” he said. “We didn’t believe the results that he was positive.”
They set out to determine what was protecting him.
Researchers call Whitney an Alzheimer’s escapee. Scientists have so far conclusively identified two others in the world who were resilient to the early-onset dementia their mutations should have caused.
Both had another mutation, Presenilin 1, and belonged to a large extended family in Colombia. They remained cognitively unimpaired for at least two decades longer than expected and died in their 70s from other illnesses.
Alzheimer’s is characterised by abnormal accumulations of two proteins in the brain: amyloid, which starts clumping into plaques at least 20 years before symptoms emerge, and tau, which forms tangles after amyloid accumulate. Tau is much more correlated with cognitive decline.
The brains of both Colombian “escapees” were laden with amyloid but had little tau in regions associated with Alzheimer’s, said Yakeel Quiroz, a neuropsychologist at Boston University.
Whitney’s brain is full of amyloid, probably even more than other mutation carriers in his family because he has lived so long, said Dr Jorge Llibre-Guerra, a neurologist at Washington University who coauthored a recent study on Whitney’s case. But he has very little tau.
“He’s resistant to tau aggregation and tau spread,” said Llibre-Guerra, who helps lead DIAN’s clinical trials. “That’s where his resilience is.”
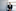
Whitney has tau accumulation in only one brain region, the left occipital lobe. That area is involved in visual-spatial functions and does not play a major role in Alzheimer’s, Llibre-Guerra said.
Quiroz said the Colombian woman’s tau accumulated in the same general area. The cases show that “people can actually have amyloid pathology without having the tau, and that amyloid is not enough to actually create a decline”, she said.
Determining how progression from amyloid buildup to tau accumulation was interrupted could provide a guide for treatment.
“They have now shown the decoupling of amyloid from tau tangles and, when that happens, the sparing of dementia,” said Kosik, who reviewed the Whitney study for Nature Medicine. “That’s where the science lies.”
Unravelling the riddle of Whitney’s resilience has revealed an intricate neurological ballet.
There is his DNA, which researchers have found includes several gene variants his afflicted relatives don’t have. Most interesting are three mutations possibly involved in neuroinflammation or tau pathology, Llibre-Guerra said.
There is Whitney’s immune system. “Your inflammatory response is lower than other mutation carriers,” Llibre-Guerra told him during the March visit, explaining that his immune system may be shielding him by not overreacting to amyloid.
And there is an especially surprising discovery: Whitney has an excess of heat shock proteins, which help keep other proteins from folding incorrectly, a defect associated with many neurological disorders.
“The levels you have are significantly higher than what you would expect,” Llibre-Guerra told him. “It may be that those proteins are preventing the misfolded proteins, especially tau, from spreading throughout the brain.”
All those factors, possibly with others that remain undiscovered, may be acting in combination to protect him, researchers said.
His case is so complex that Bateman described his team’s recent published study as “a call to arms” intended “to draw attention from other researchers to say ‘Hey, here’s a really important person, a really important case, and you need to help figure this out’”.
Scientists have not yet found Whitney’s “missing needle in the haystack and said, ‘eureka’”, Bateman said, but they will keep searching. The puzzle that protects Doug Whitney is too valuable not to be solved.
This article originally appeared in The New York Times.
Written by: Pam Belluck
Photographs by: M. Scott Brauer
©2025 THE NEW YORK TIMES
